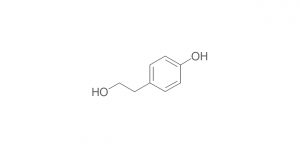
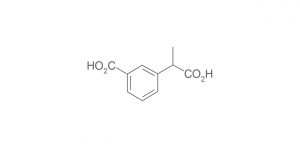
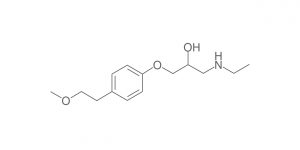
Olanzapine RC A (USP); Olanzapine Impurity A (EP)

Olanzapine RC A (USP); Olanzapine Impurity A (EP)
GA01137 - 98%
In stock
 Delivery:
3 to 5 working days
Delivery:
3 to 5 working days
Others quantities on request
Specifications
- CAS RN: 138564-59-7
- Purity: 98%
- Mol Formula: C12H9N3O2S
- Mol Weight: 259.29
- Tags: Pharmaceutical Reference Substances and Impurities ; Olanzapine ;
-
Description
GA01137
Olanzapine (originally branded Zyprexa) is an atypical antipsychotic. It is approved by the U.S. Food and Drug Administration (FDA) for the treatment of schizophrenia and bipolar disorder. It is structurally similar to clozapine and quetiapine. It is a dopamine antagonist and is classified as a thienobenzodiazepine.
We have successfully synthesized impurities of the Olanzapine. This will be of interest to pharmaceutical companies and analytical labs.
-
Additional information
Packing Unit 500 mg, 1 g